Developing OmniSomes as First-in-Class Regenerative Gene Therapies
A novel inhaled stem cell Extracellular Vesicle (exosome) based platform technology
OmniSome Therapeutic Platform
EV (Exosome) Genetic Cargo can be Tailored to Treat Virtually any Disease
OmniSomes lung targeting technologies offer an exemplar delivery platform for treating respiratory diseases and are the initial focus of the company’s therapeutic pipeline (see below). The first problem to solve for pulmonary gene therapy is that the gene transfer vectors must efficiently reach the lung via aerosol delivery. Our partnership with Aerogen Limited has resulted in optimised OmniSome formulations with their state-of-the art vibrating mesh nebuliser technology – delivering OmniSomes with high efficiency while maintaining the structural integrity and functionality of the EVs.
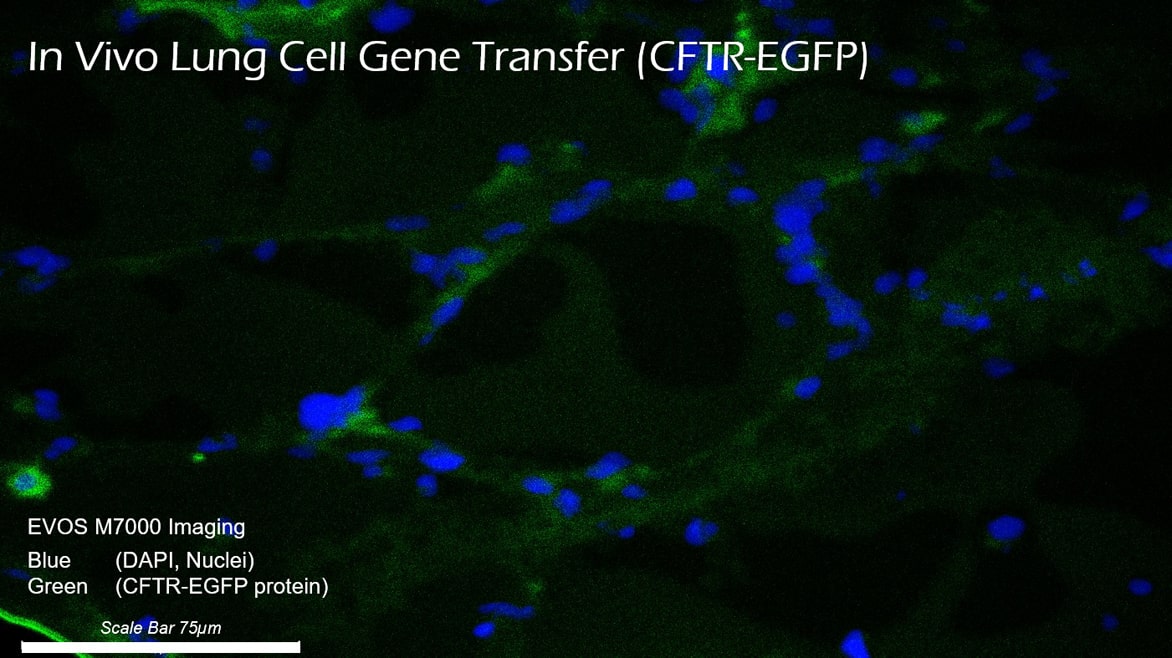
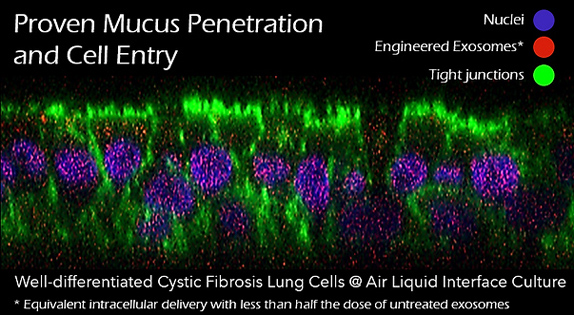
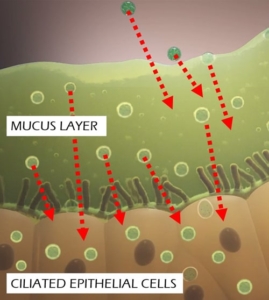 Trapping of delivery vectors in viscous mucus in the lung is another significant barrier to gene transfer to the underlying target cells. Even if the mucus layer can be penetrated, inefficient penetration through the cell membrane further impedes access of traditional gene therapy vectors to the underlying target cells, thus preventing successful gene transfer. OmniSomes have exhibited mucus diffusion and tropism for cell entry and gene transfer in human lung cell models (air-liquid interface cell cultures) and have been successfully aerosolised in small animal studies to effect in vivo gene transfer.
Trapping of delivery vectors in viscous mucus in the lung is another significant barrier to gene transfer to the underlying target cells. Even if the mucus layer can be penetrated, inefficient penetration through the cell membrane further impedes access of traditional gene therapy vectors to the underlying target cells, thus preventing successful gene transfer. OmniSomes have exhibited mucus diffusion and tropism for cell entry and gene transfer in human lung cell models (air-liquid interface cell cultures) and have been successfully aerosolised in small animal studies to effect in vivo gene transfer.
Adding further complexity is the processing of the carriers inside the cells – EVs are naturally occurring carriers of RNA and other messengers from cell to cell in the body – this gene transfer, refined by millennia of evolution, has demonstrated 30-40 fold more efficient gene transfer than synthetic lipid nanoparticles*.
The benefits of the OmniSome technology are not just related to delivery and efficient gene transfer as they are derived from stem cells which have widely documented therapeutic efficacy – they are thus expected to further benefit patients afflicted by respiratory diseases through anti-inflammatory, anti-fibrotic, anti-microbial, immune cell modulatory and tissue regeneration effects. The stem cells used in research and product development are ethically sourced from healthy, consenting donors (bone-marrow or adipose tissues).
* Murphy DE, de Jong OG, Evers MJW, Nurazizah M, Schiffelers RM, Vader P. Natural or Synthetic RNA Delivery: A Stoichiometric Comparison of Extracellular Vesicles and Synthetic Nanoparticles. Nano Lett. 2021 Feb 24;21(4):1888-1895. doi: 10.1021/acs.nanolett.1c00094. Epub 2021 Feb 11. PMID: 33570966; PMCID: PMC8023702.
Partnering Opportunities
The properties of OmniSomes are applicable to a wide variety of diseases amenable to gene therapy, targeting other organs in the body (e.g., liver, CNS, digestive and cardiovascular systems).
Feel free to contact us to discuss how OmniSomes could enable the partnered co-development of your RNA therapeutic assets by leveraging our expertise in EV manufacturing and delivery technologies.
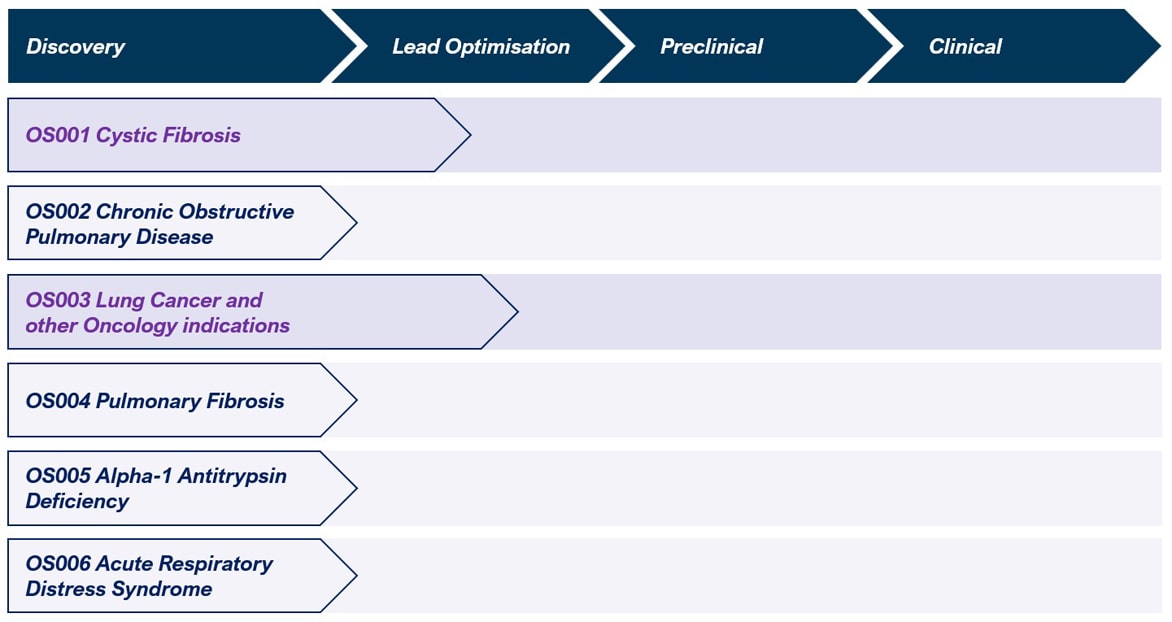
OmniSome Pipeline
Exosomes Exhibit Unparalleled Intracellular Delivery and Therapeutic Properties
Delivery, delivery, delivery are the often cited biggest problems associated with unlocking the transformative potential of gene therapies. Genetic medicine approaches such as mRNA, gene silencing, gene editing and DNA gene therapies have curative potential for a host of disease indications previously untreatable. They have yet to enter the mainstream, due to safety concerns, complex manufacturing & difficulties delivering them efficiently to areas other than the liver, kidney & circulatory system.
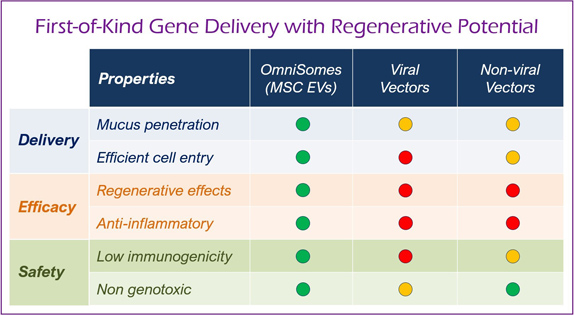
The OmniSome platform piggybacks on the natural abilities of stem cell extracellular vesicles to efficiently deliver RNA cargoes from cell to cell and addresses difficulties related to safe & effective gene transfer via traditional approaches using viral vectors (e.g., lentiviruses & AAVs) and non-viral vectors (e.g., liposome and lipid, protein, and polymeric nanoparticles). Stem cell EVs have regenerative medicinal properties and are highly advantageous compared to other EVs based carriers (e.g., algae/milk derived EVs and HEK293 cells).
Competing viral and non-viral gene transfer vectors are inefficient & can cause inflammation, immunogenicity & elicit poor functional gene transfer.
OmniSomes lung targeting technologies offer an exemplar delivery platform for treating respiratory diseases and are the focus of the company’s therapeutic pipeline. The first problem is that gene transfer vectors must reach the lung via aerosol delivery. Trapping of the vectors in viscous mucus in the lung is a significant barrier to gene transfer to the underlying target cells. Even if the mucus layer can be penetrated, inefficient penetration through the cell membrane further impedes access of these vectors to the underlying target cells, thus preventing successful gene transfer. OmniSomes have exhibited mucus diffusion and tropism for cell entry and gene transfer in human lung cell models (air-liquid interface cell cultures) and have been successfully aerosolised in small animal studies to effect gene transfer (see images below). The benefits of the OmniSome technology are not just related to delivery and efficient gene transfer as they are derived from stem cells which have widely documented therapeutic efficacy – they are thus expected to further benefit patients afflicted by respiratory diseases through anti-inflammatory, anti-fibrotic, anti-microbial, immune cell modulatory and tissue regeneration effects. OmniSomes have a unique capacity to regenerate and repair the functional capacity of the diseased damaged lung, repair is essential to improve lung function which becomes severely impaired in people with chronic inflammatory lung diseases. The stem cells used in research and product development are ethically sourced from healthy, consenting donors (bone-marrow or adipose tissues).